Abstract
INTRODUCTION: Myelodisplastic syndrome (MDS) is an aging associated clonal hematopoietic disorder characterized by a bone marrow failure in which immature blood cells do not mature properly. Oxidative stress seems to play a role in its pathogenesis. Reactive oxygen species (ROS) regulation exert a control between self-renewal, proliferation and cell differentiation. Thus, ROS imbalance could lead to disease, especially in bone marrow where cells seems to be vulnerable to oxidative stress. Interestingly, high ROS levels have been observed in MDS, which has been correlated with a diminished survival. Several types of molecules can be directly affected by oxidative damage. However, the oxidation of proteins is of particular concern since it leads to aggregation, unfolding, or conformational changes that may confer a loss of structural or functional activity. As an altered proteome has also been described during MDS, in this study we have analyzed the pattern of oxidized protein associated to MDS. Moreover, we analyzed whether MDS patients could be beneficed by the antioxidant effects of Deferasirox therapy.
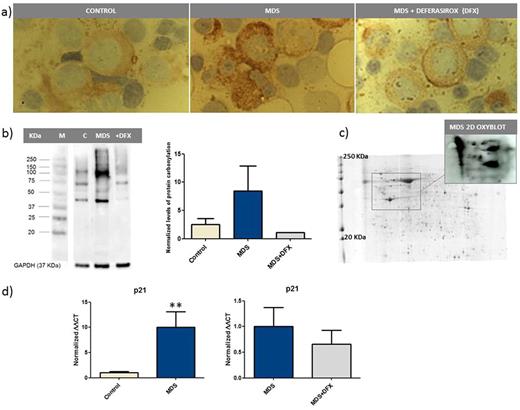
METHODS: Protein carbonylation was analyzed in bone marrow (n=14; 8 MDS and 6 controls) by immunohistochemistry after derivatization of carbonyl compounds with 2,4-dinitrophenylhydrazine (DNPH) and specific antibody detection of the 2,4-dinitrophenyl hydrazones. Protein carbonylation was also studied in expanded erythroblasts during in vitro erythropoiesis (n=9; 5 MDS and 4 controls) by 1-D and 2-D immunoproteomics. p21 mRNA levels were analysed by qRT-PCR using Taqman probes in a Applied Biosystems 7500 RT-PCR System. β-glucuronidase gene was used as endogenous reference to normalize data. Differences in expression mean values between the groups were analyzed using T-student or Mann-Whitney U test.
RESULTS: Immunohistochemistry assays showed a higher level of carbonylation in MDS samples (Figure 1a). We observed carbonylation in the cytoplasm of myeloid cells, whereas erythroid cells remained negative in both, patients and controls. Interestingly, MDS patients treated with Deferasirox presented lower levels than non-treated patients. Further, to assess carbonylation in the erythroid precursors, we analyzed the pattern of carbonylated proteins in expanded erythroblasts. Again, a higher level was observed in MDS vs . control group, which was reverted after Deferasirox treatment (Figure 1b). Most carbonylated proteins ranged in a size between 40-250 KDa. A highly carbonylated 40 KDa band was detected in all analyzed SMD samples. As seen in the 2D immunoblots, these differences in carbonylation were caused by a limited number of proteins or aggregates, rather than to a large spectrum of proteins (Figure 1c). Finally, as oxidative stress signaling pathways could regulate the cell cycle by p53, we analyzed the expression of p21, a p53 target during stress response. Upregulation of p21 was significantly observed in MDS samples. Remarkably, this effect was reverted upon Deferasirox treatment (Figure 1d).
CONCLUSION: An altered protein carbonylation pattern of myeloid and erythroid lineages was observed in MDS patients. This boosted oxidative cellular environment seems to activate signaling pathways involving p21. Deferaxirox treatment partially reverted these oxidative lesions at cellular level.
*both authors have contributed equally
M.L. hold a postdoctoral Fellowship of the Spanish Ministry of Economy and Competitiveness (FPDI-2013-16409)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal